The language of humiliation is a step towards a second civil war, argues RAMZY BAROUD
 [Centre for Geobiology/University of Bergen Norway/CC]
[Centre for Geobiology/University of Bergen Norway/CC]
LIVING things are astonishingly varied. From the flapping wings of a bird to the pulsating of a jellyfish, the croak of an Amazonian toad to a human cry for joy, the diversity of life is what attracts many people to study biology.
However, beneath that diversity lies a remarkable similarity. Living things can be divided into three domains. Like all other multicellular lifeforms, humans are eukaryotes: our cells have a very different structure to the other two major domains of life — the single-celled bacteria and the archaea — most notably a cell nucleus.
Eukaryotes are more recent organisms, but they are still ancient. Though molecular dating is an imprecise process, scientists are pretty certain that eukaryotes evolved more than 1.5 billion years ago. How they emerged is a matter of huge debate, but the leading theory is that they arose from a fusing together of an organism from the archaea with a bacterium.
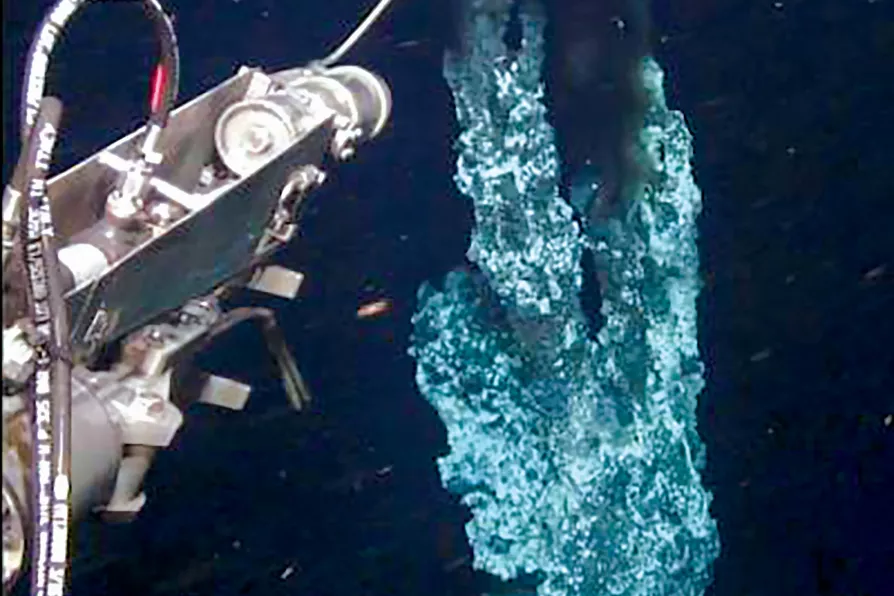
The closest relatives of the ancestral archaea that gave rise to the eukaryotes are believed to be the Asgard archaea. They were given their mythological name because the first representatives were isolated from a hydrothermal vent over two kilometres deep in the Arctic ocean called Loki’s Castle; subsequent related archaea were named after Thor, Odin and Heimdall.
Until 2020, scientists were not able to culture the Asgard archaea to examine them in the laboratory because they couldn’t replicate deep-sea conditions. The Asgard archaea were known only by their genes. It’s fair to say that by sight alone you would not link a tiny blob of a cell from deep-sea sediment with the complexity of a human body. And even on a cellular level, human cells look quite different to the Asgard archaea.
However, observations on a smaller scale — that of molecules — show overwhelming evidence for our relatedness. The basic machinery of life — DNA and other aspects of molecular biology — has remained unchanged over all that time, even as evolution has led to more and more diverse life forms. As the French scientist and socialist Jacques Monod put it, “natural selection alone and unaided … [has] drawn all the music of the biosphere.”
This month, new research published in Nature Ecology and Evolution adds yet another powerful piece of evidence of the shared ancestry of life on Earth. The story begins with the discovery in 2001 of a protein in humans called viperin, which seemed to be part of the immune response to infection by viruses when they burrow into our cells.
Researchers discovered that viperin works by stopping the virus from replicating its genetic material, which it achieves by producing a small molecule that acts like a “cancel” signal.
However, it became clear that viperin is not only a feature of human immunity. Just as humans can get viruses, so too can single-celled organisms like bacteria and archaea. And further, they also have similar proteins to human viperin. Although less common than in eukaryotes, the proteins are all known as viperins.
It was clear that if they were present in both humans and archaea that the viperins must have a shared ancestry going back over billions of years, but we knew little about it. The new research this month, which was led by Aude Bernheim in France and Fabai Wu in China, tried to explore it in more detail.
First, the researchers searched modern-day archaea for genes that defend against viruses. They found that the viperin genes were much more common in the Asgard archaea than in other archaea or bacteria. Because viperins are more common in eukaryotes, this suggests that the eukaryotes all inherited them from a common ancestor, rather than gaining them separately several times.
The researchers tested the viperin genes from archaea and eukaryotes by putting them into E. coli, to see if they could protect it against infection by a bacterial virus. Amazingly, the viperins have stayed similar enough through their whole history of evolution that nearly half of them worked. Viperins from a bright green bird from Puerto Rico, a saucer-shaped jellyfish, and an Amazonian toad were among those that still worked when transplanted into E. coli.
The researchers could look carefully at the parts of the protein that had changed. By looking at the predicted structure of viperins from organisms today, they could ascertain how they must have evolved over time in their ancestors. In particular, they found the areas of the protein that had changed in eukaryotes, which allowed the viperins to evolve new functions.
Once the eukaryotes had emerged, there seems to have been a duplication of the viperin gene very early in their evolution. Having two copies of a gene allows for diversification — one copy can keep the original function, while the other can vary, and through mutation change its function.
The viperins also interacted with other proteins. Human viperin works in tandem with another protein, encoded next to it in the genome, which enhances its own activity. This close partnership is conserved across eukaryotes, which is rare, although such partnerships are common in single-celled organisms. That suggests that eukaryotes may have inherited them that way and are under strong selective pressure to keep them close together.
As the eukaryotes evolved into many different groups of organisms, the viperins started to get used in slightly different ways and linked to other genes. As the researchers put it, over time the viperin genes formed “new partnerships,” including getting totally fused together with other genes to make new ones. Viperins have had time to develop a whole variety of associations. Yet despite all these opportunities for change, by and large the viperins still function in a remarkably similar way.
These recent findings are a vivid reminder of the relatedness of all living things. Who would have thought that a protein that helps a fish protect itself against fish-viruses could help a bacterium? As Monod put it, sometimes one feels that the most amazing thing about biology is not evolution, but life’s stability in the face of constant change; not its diversity, but rather its lack of it.
New research into mutations in sperm helps us better understand why they occur, while debunking a few myths in the process, write ROX MIDDLETON, LIAM SHAW and MIRIAM GAUNTLETT

GEORGE FOGARTY falls under a spell of an unpretentious gathering that is as edifying as it is entertaining











