The US-Israeli strikes against Iran are part of a decades-long war against the Islamic Republic which has refused to bow to US demands that it surrender its sovereignty, argues VIJAY PRASHAD
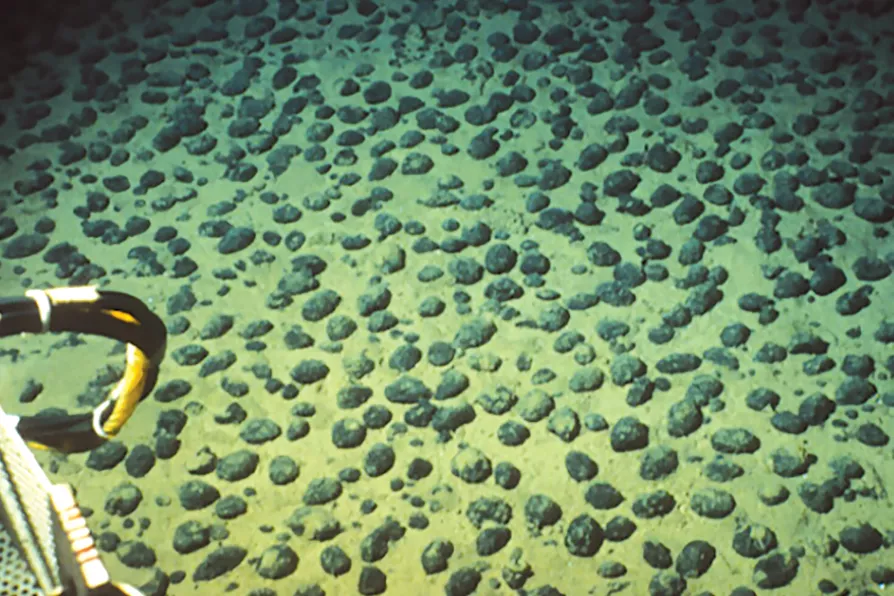
 MORE QUESTIONS THAN ANSWERS: Field of polymetallic nodules of type C in the north equatorial Pacific, photo taken from the submarine Nautile - NODINAUT campaign. The formations can be found as deep as 20,000 feet below the ocean's surface
[Philweb/CC]
MORE QUESTIONS THAN ANSWERS: Field of polymetallic nodules of type C in the north equatorial Pacific, photo taken from the submarine Nautile - NODINAUT campaign. The formations can be found as deep as 20,000 feet below the ocean's surface
[Philweb/CC]
SOME astrophysicists try to understand the composition of planets that go round other stars — so-called “exoplanets.” They do so by looking at how light from the star is absorbed as the planet orbits around it, which allows them to find out the compounds that make up the planet’s atmosphere.
The technique allows scientists to understand something about these distant planets, though they are separated by many light years.
Some scientists believe that by identifying specific molecules in the atmospheres of these planets, we will be able to observe the signature of life. These signatures would be “biomarkers” — molecules that can’t be produced by simple chemistry, but can only be made by living organisms.
The difficulty in assessing these molecules and the remoteness of the planets make others sceptical that any biomarker would, by itself, ever be enough to categorically say that life exists.

Neutrinos are so abundant that 400 trillion pass through your body every second. ROX MIDDLETON, LIAM SHAW and MIRIAM GAUNTLETT explain how scientists are seeking to know more about them

Olive oil remains a vital foundation of food, agriculture and society, storing power in the bonds of solidarity. Though Palestinians are under attack, they continue to press forward write ROX MIDDLETON, LIAM SHAW and MIRIAM GAUNTLETT

200 years since the first dinosaur was described and 25 after its record-breaking predecessor, the BBC has brought back Walking with Dinosaurs. BEN CHACKO assesses what works and what doesn’t
Science has always been mixed up with money and power, but as a decorative facade for megayachts, it risks leaving reality behind altogether, write ROX MIDDLETON, LIAM SHAW and MIRIAM GAUNTLETT










