MBDA’s Alabama factory makes components for Boeing’s GBU-39 bombs used to kill civilians in Gaza. Its profits flow through Stevenage to Paris — and it is one of the British government’s favourite firms, reveals SOLOMON HUGHES
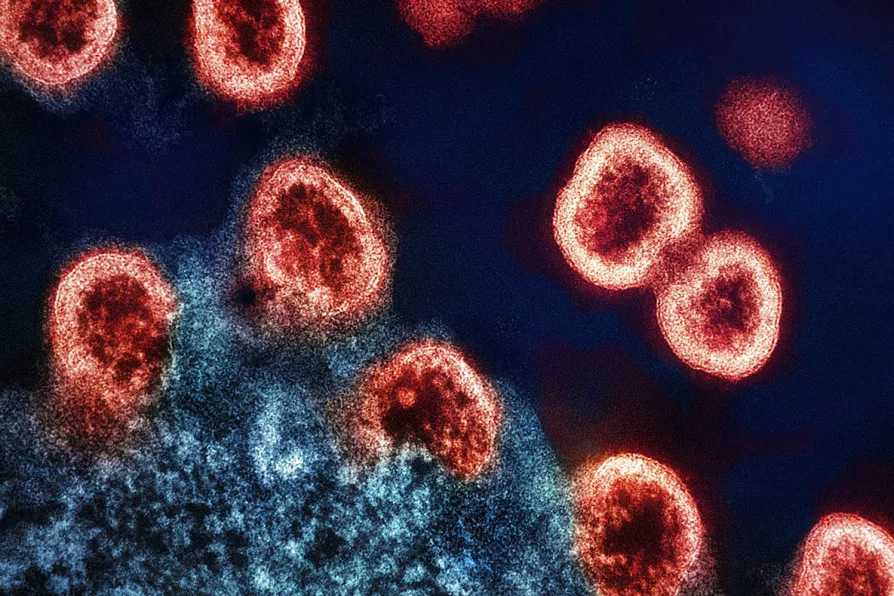
 An electron micrograph of HIV-1 virus particles (colourized red) replicating from an HIV-infected H9 T-cell (blue)
An electron micrograph of HIV-1 virus particles (colourized red) replicating from an HIV-infected H9 T-cell (blue)
LENACAPAVIR, an injectable antiretroviral drug developed by Gilead Sciences, recently made headlines after a Phase 3 clinical trial in South Africa and Uganda showed it to be 100 per cent effective in preventing HIV among women and adolescent girls.
Though hailed as a breakthrough in HIV prevention, lenacapavir serves as a stark reminder of the problems with the pricing of life-saving medicines.
While further data from this study and results from studies involving other populations are needed, lenacapavir could be considered the most durable HIV prevention method to show efficacy among women — a population for who biomedical HIV prevention evidence has been severely limited.

When privatisation is already so deeply embedded in the NHS, we can’t just blindly argue for ‘more funding’ to solve its problems, explain ESTHER GILES, NICO CSERGO, BRIAN GIBBONS and RATHI GUHADASAN













